BIOSOLVE-IV FULL COHORT
24m follow-up of full cohort with 2,066 patients1
BIOSOLVE-IV 2-year results
Conclusions
- At 24m of follow-up the Magmaris® Resorbable Magnesium Scaffold (RMS) scaffold showed excellent safety and efficacy profile
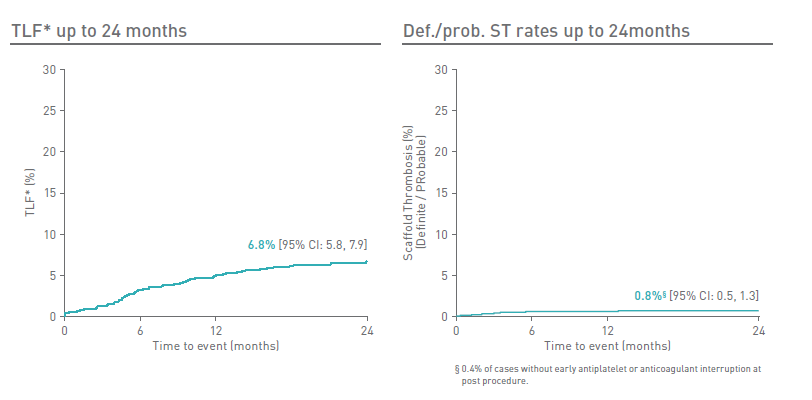
- Target Lesion Failure (TLF)* rate at 24m is comparable to contemporary newer generation drug-eluting stents2,3,4
- Definite/probable scaffold thrombosis rate at 24m is 0.8%
- 0.4% scaffold thrombosis rate excluding cases with early antiplatelet or anticoagulant interruption