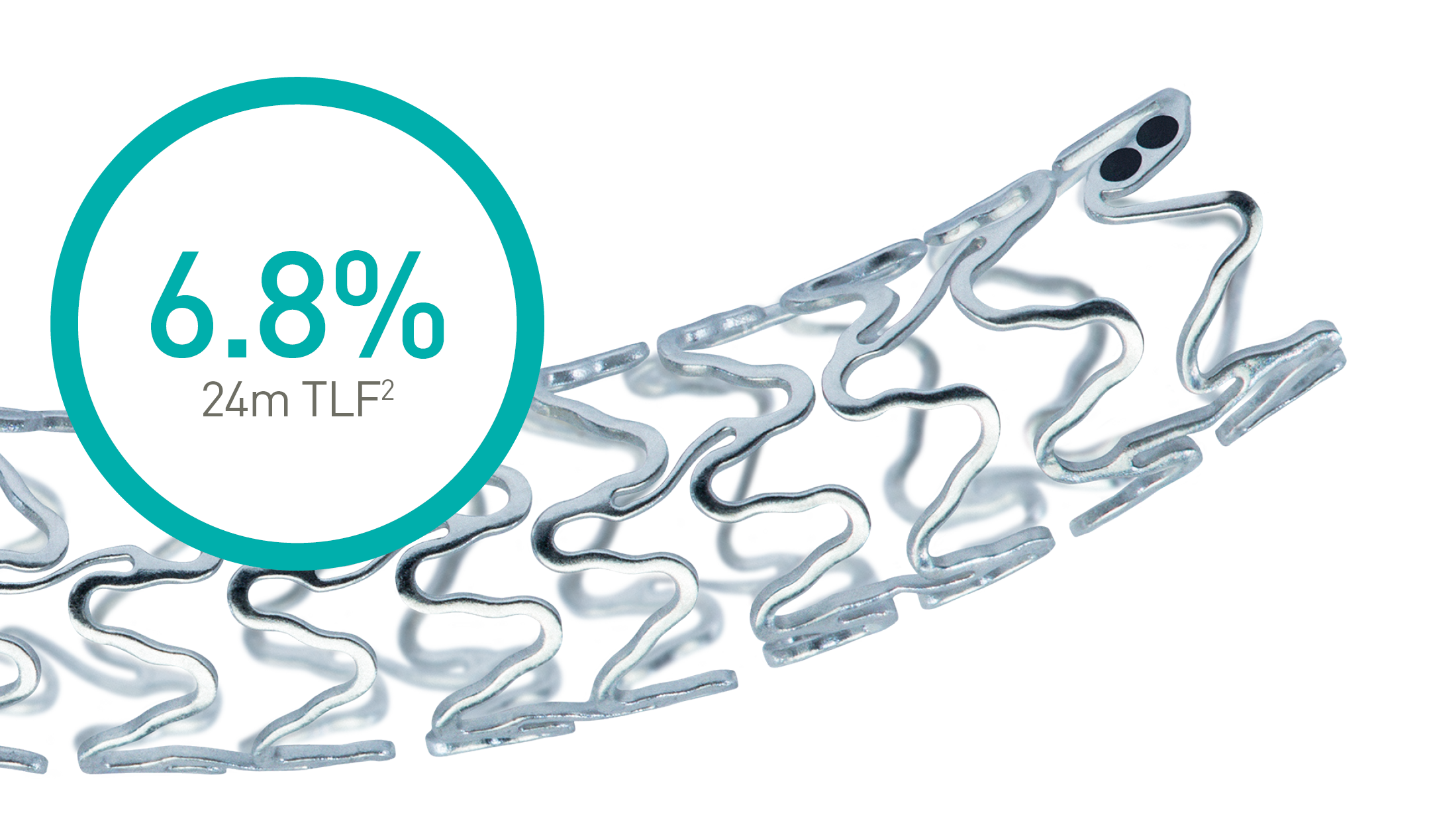
Magmaris® RMS
Confirmed clinical safety and,
efficacy*, fast Magnesium
resorption and
better deliverability.
Procedure
Experience shows1 that following the 4 Ps implantation strategy for
Magmaris may lead to better patient outcomes.
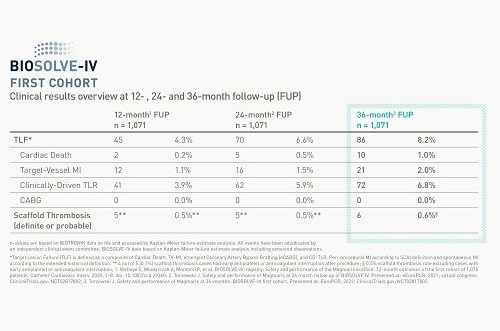
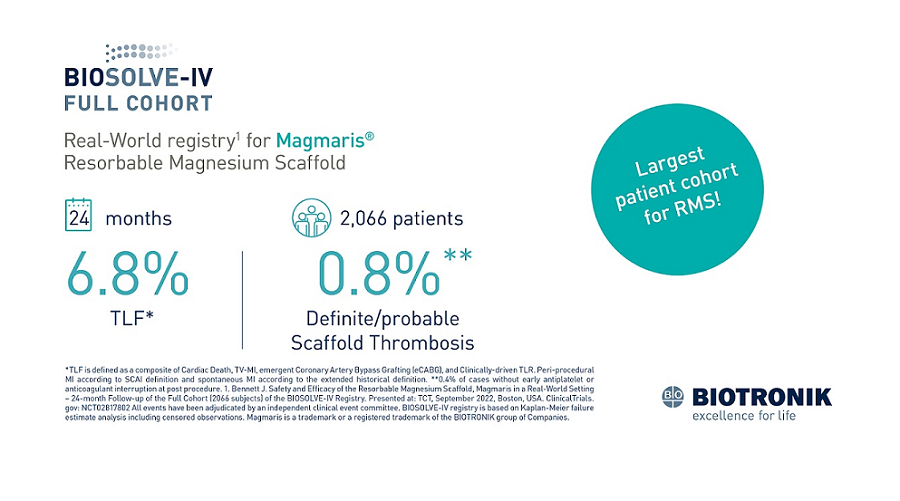
Clinically Proven
Magmaris’ clinical body of evidence keeps growing with new data from BIOSOLVE-IV