Support
DES-like vessel support*

Resorb
~95% of Magnesium is resorbed at 12 months1

Restore
Lowers the risk of neoatherosclerosis**, a known risk for scaffold thrombosis†

Support
DES-like vessel support*

Resorb
~95% of Magnesium is resorbed at 12 months1

Restore
Lowers the risk of neoatherosclerosis**, a known risk for scaffold thrombosis†
TLF and ST rates in BIOSOLVE studies remain low and comparable to 2nd generation DES out to 36 months in similar patient population.


* Tested up to 28 days in pre-clinical trials.
** As demonstrated in pre-clinical studies.
† Very late.
†† TLF defined as a composite of cardiac death, Target-Vessel MI and Clinically-Driven TLR.
No recoil increase is measured after one hour.7
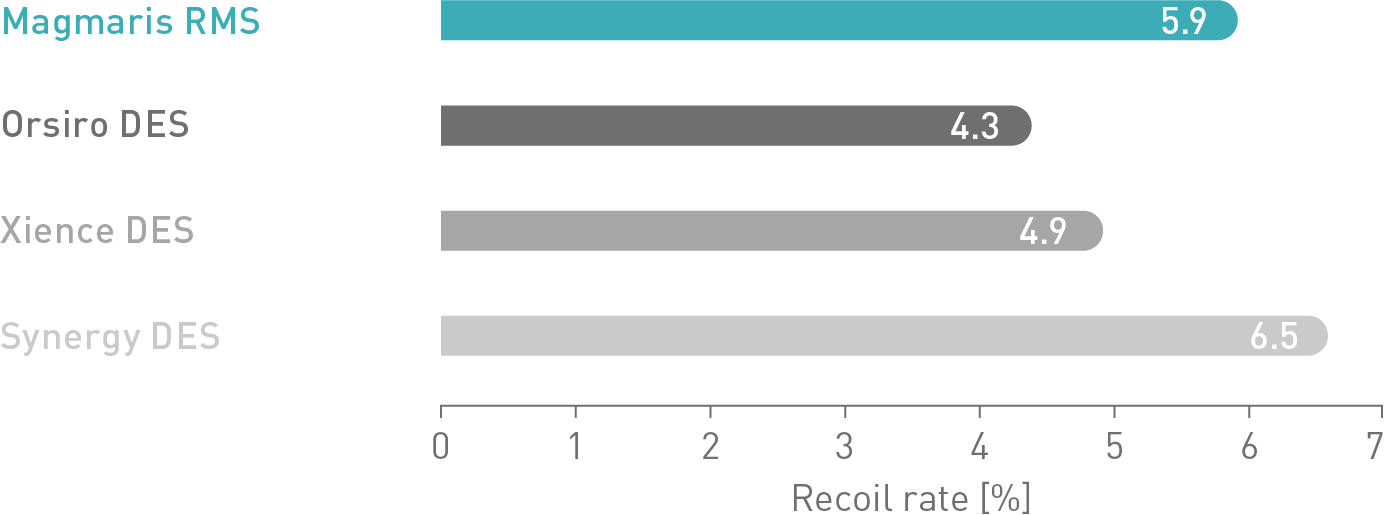
The recoil rate is measured by calculating the difference between the diameter of the device at nominal pressure and the diameter of the device when no pressure is applied.
Multivariate adjustments showed that the device type was not an independent predictor of clinical adverse events.8
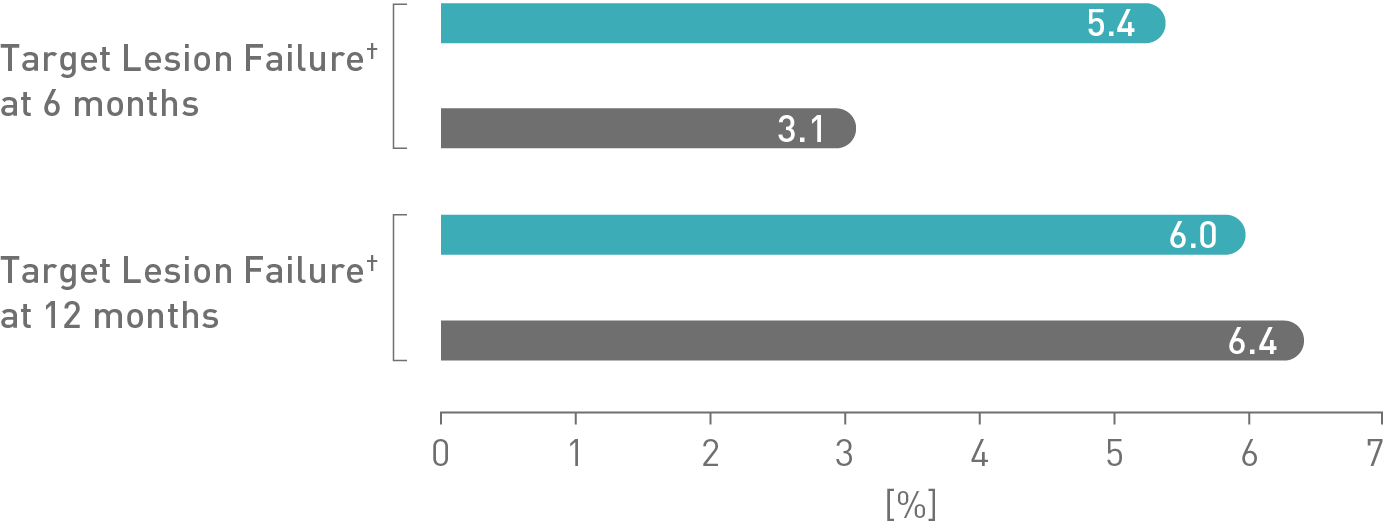
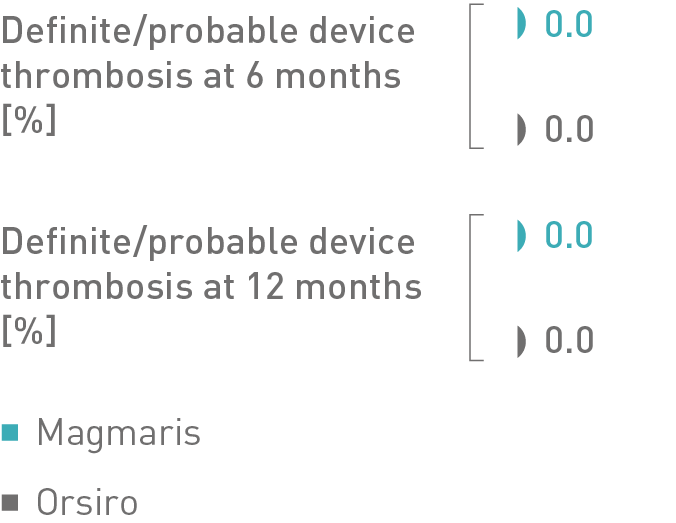
Propensity matched analysis comparing Magmaris to Orsiro at 12-month follow-up.
* Tested up to 28 days in pre-clinical trials.
† TLF defined as a composite of cardiac death, Target-Vessel MI and Clinically-Driven TLR.

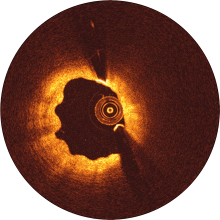
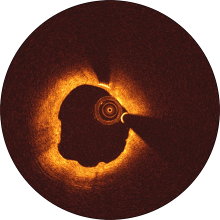



Magmaris and the custom-made stainless steel DES were implanted in a silicone tube and exposed to porcine blood flow for 1 hour.
The test was repeated with different positions for each device. After 1 hour, platelet coverage of the devices’ surfaces was analyzed by immunostaining.
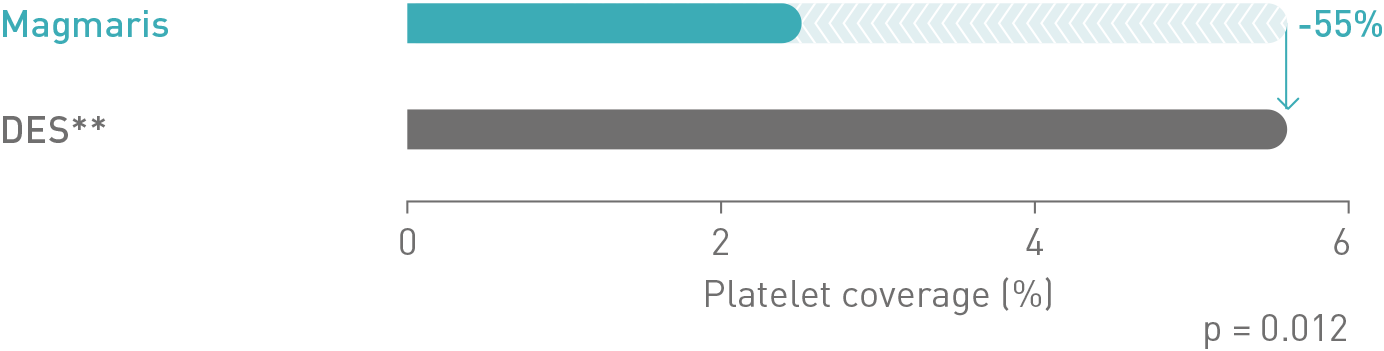

Magmaris

DES**
40% of patients who died 9 months after a DES implantation showed signs of neoatherosclerosis.11
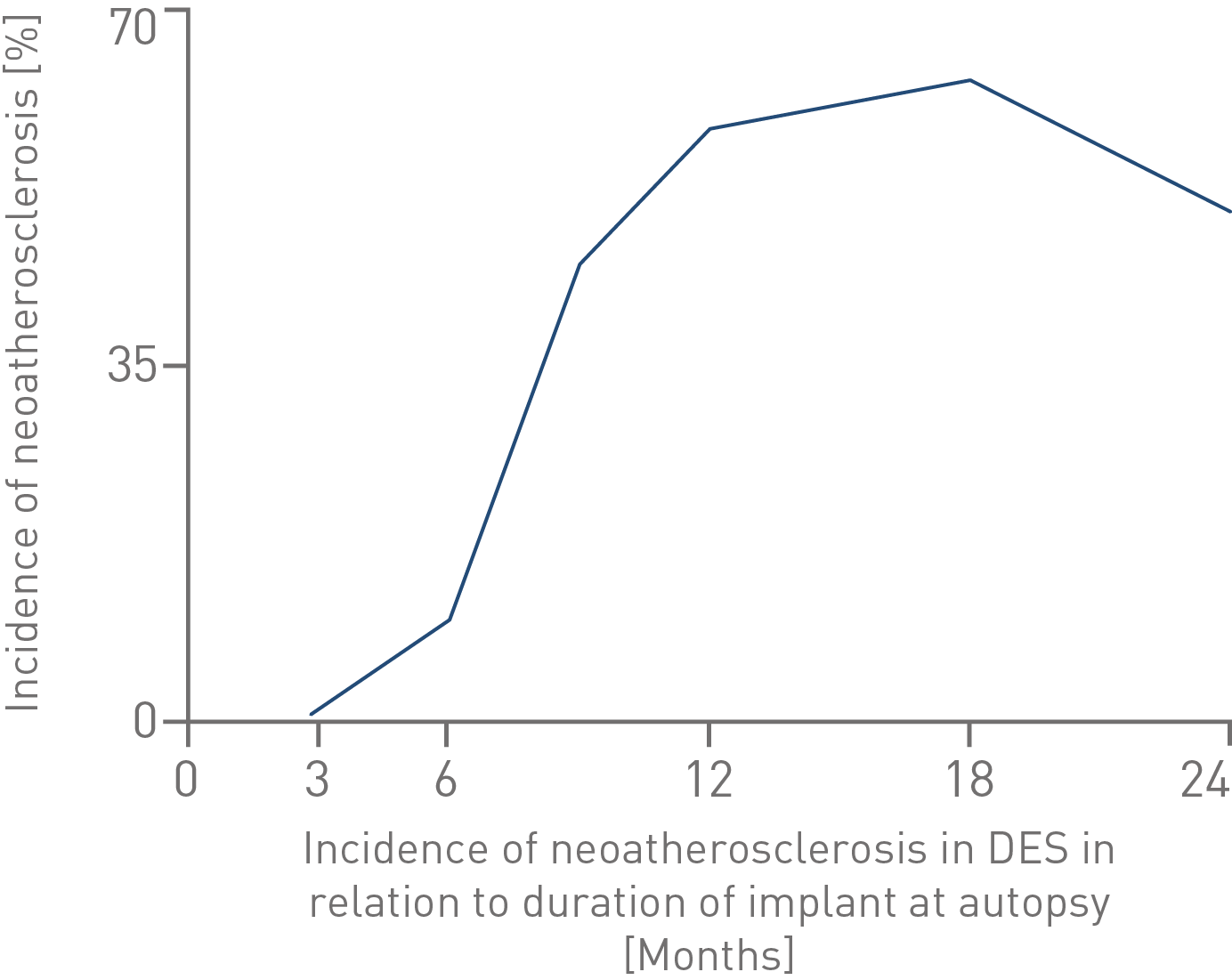
* As demonstrated in pre-clinical studies.
** Custom-made stainless steel DES with the same Magmaris design and coating.
† Neoatherosclerosis is defined as the phenomenon of the transformation of stent neointima from normal neointima to an atherosclerotic lesion.
Device implantation
Endothelialization starts
Leaky endothelium allows macrophages and cholesterol infiltration
Foamy macrophages contribute to plaque development, e.g. neoatherosclerosis
Plaque ruptures eventually leading to device thrombosis
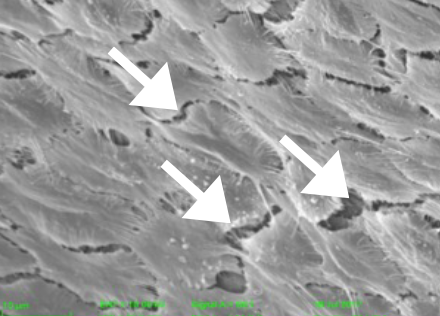
In a rabbit model, Magmaris shows tightly formed endothelial cell junctions compared to a custom-made stainless steel DES**.
Tightly packed endothelial cells reduce macrophages and cholesterol infiltration which in turn potentially reduces the risk of neoatherosclerosis, a predictor of very late events.
Greater endothelial integrity is associated with lower macrophages infiltration.13
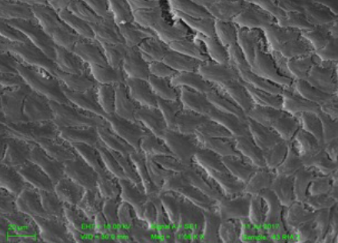
Magmaris
DES**
Arrow-heads indicate leaky endothelial junctions
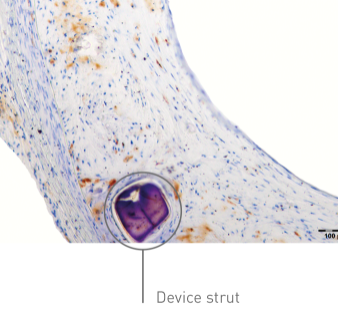
The accumulation of foamy macrophages in the neo-intimal space is an early sign of neoatherosclerosis.
Mean macrophage score reduction Magmaris vs. DES**: p < 0.0001
Magmaris
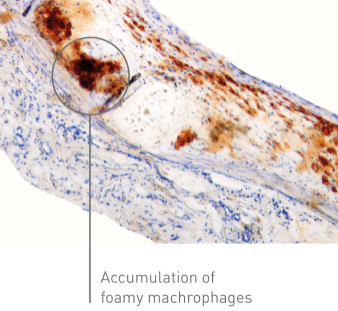
DES**
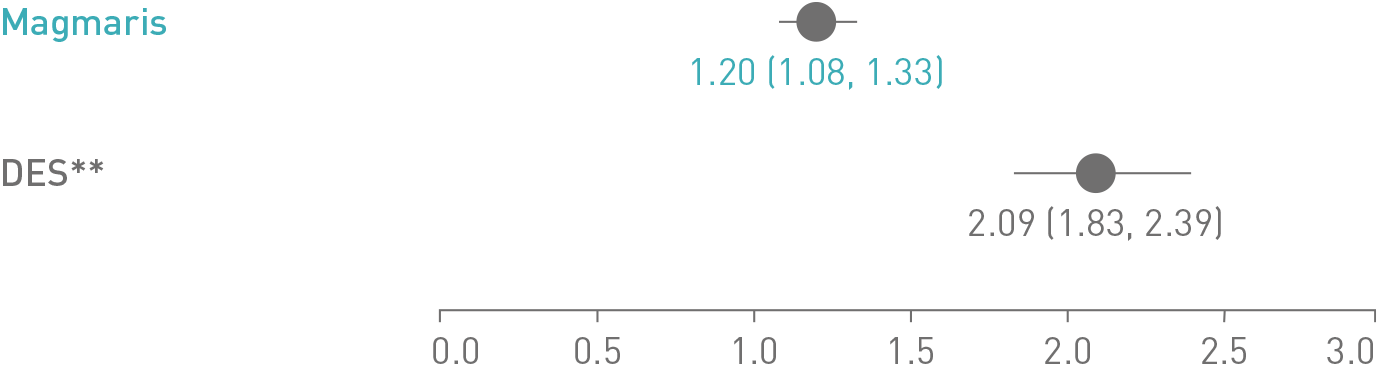
Magmaris significantly reduces the mean neoatherosclerosis score compared to a DES**, 12
Mean neoatherosclerosis score reduction Magmaris vs. DES**:
p < 0.0001
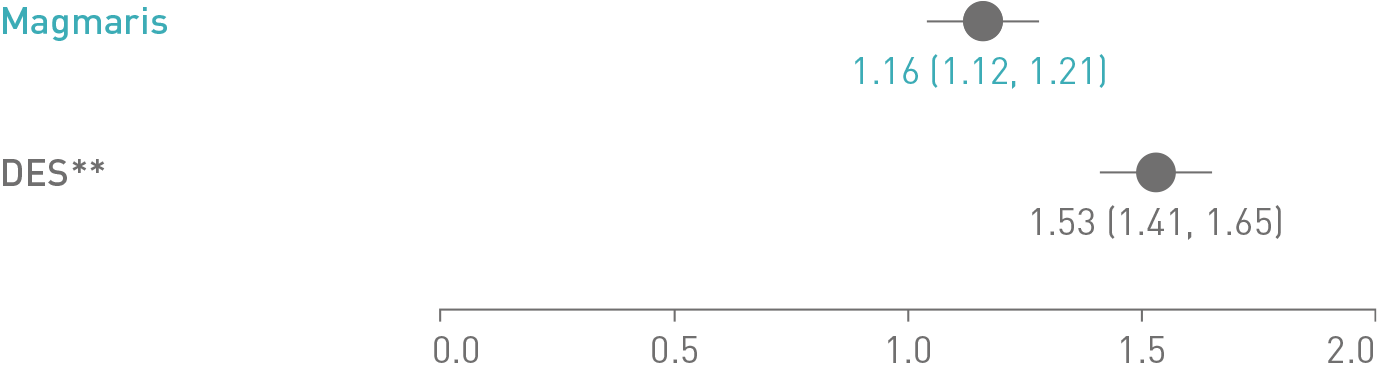
The neoatherosclerosis score was assessed by histology and determines the quantity of foam cells in different layers of the neointima.
Magmaris |
24 months (Full Cohort) BIOSOLVE-IV1Full cohort (n=2,066) 6.8%TLF◊ |
0.8%ΔDefinite/probable scaffold thrombosis |
|
36 months (First Cohort) BIOSOLVE-IV2First cohort (n=1,075) 8.2%TLF◊ |
0.6%oDefinite/probable scaffold thrombosis |
|
|
36 months BIOSOLVE-II/-III3 (n=184) 6.3%TLF◊ |
0.0%Definite/probable scaffold thrombosis |
|
|
60 months BIOSOLVE-II4 (n=123) 8.0%TLF◊ |
0.0%Definite/probable scaffold thrombosis |


36 months BIOSOLVE-II17 (n=21)
Progression
Regression
Stable
Progression/regression defined as +/- 5% change from baseline
All n-values represent the actual number of patients enrolled.* Based on BIOSOLVE-II, -II/-III and -IV, for patient populations see study details.◊ Target Lesion Failure (TLF) defined as a composite of Cardiac death, Target-Vessel Myocardial Infarction (TV-MI),emergent Coronary Artery Bypass Grafting (eCABG), and Clinically-Driven Target Lesion Revascularization (CD-TLR).° 0.5% scaffold thrombosis rate excluding cases with early antiplatelet or anticoagulant interruption.Δ 0.4% scaffold thrombosis rate excluding cases with early antiplatelet or anticoagulant interruption.
1. Joner M, Ruppelt P, Zumstein P, et al. Preclinical Evaluation of Degradation Kinetics and Elemental Mapping of First and Second Generation Bioresorbable Magnesium Scaffolds. EuroIntervention. 2018 Feb 20. pii: EIJ-D-17-00708. doi: 10.4244/EIJ-D-17-00708; 2. Haude M, Ince H, Kische S, et al. Safety and clinical performance of a drug eluting absorbable metal scaffold in the treatment of subjects with de novo lesions in native coronary arteries: Pooled 12-month outcomes of BIOSOLVE-II and BIOSOLVE-III. Catheter Cardiovasc Interv. 2018;00:1–10. doi: 10.1002/ccd.27680; 3. Haude M, Ince H, Kische S, et al. Sustained safety and performance of the second-generation sirolimus-eluting absorbable metal scaffold: Pooled outcomes of the BIOSOLVE-II and -III trials at 3 years. Cardiovascular Revascularization Medicine. 2020. doi: 10.1016/j.carrev.2020.04.006; 4. Dangas G, Serruys P, Kereiakes D, et al. Meta-Analysis of Everolimus-Eluting Versus Paclitaxel-Eluting Stents in Coronary Artery Disease. Final 3-Year Results of the SPIRIT Clinical Trials Program (Clinical Evaluation of the Xience V Everolimus Eluting Coronary Stent System in the Treatment of Patients With De Novo Native Coronary Artery Lesions). JACC: Cardiovascular Interventions. 2013; 6/9; doi: 10.1016/j.jcin.2013.05.005; 5. EVOLVE FHU 24m: Meredith I, Verheye S, Weissmann N, et al. Six-month IVUS and two-year clinical outcomes in the EVOLVE FHU trial: a randomised evaluation of a novel bioabsorbable polymer-coated, everolimus-eluting stent. EuroIntervention. 2013; 9: 308-315 and Meredith I, Verheye S, Dubois C et al. Final five-year clinical outcomes in the EVOLVE trial: a randomised evaluation of a novel bioabsorbable polymer-coated, everolimus-eluting stent. EuroIntervention 2018;13:2047-2050. doi: 10.4244/EIJ-D-17-00529; 6. BIOTRONIK data on file; 7. Schmidt W, Behrens P, Brandt-Wunderlich C, et al. In vitro performance investigation of bioresorbable scaffolds - Standard tests for vascular stents and beyond. Cardiovasc Revasc Med. 2016;17(6):375-83. doi: 10.1016/j.carrev.2016.05.001; 8. Hideo-Kajita A, Garcia-Garcia HM , Kolm P, et al. Comparison of clinical outcomes between Magmaris and Orsiro drug eluting stent at 12 months: Pooled patient level analysis from BIOSOLVE II–III and BIOFLOW II trials. International Journal of Cardiology. 2019: 1-30. doi: 10.1016/j.ijcard.2019.11.003; 9. BIOSOLVE-II case, GER443-012. Courtesy of M. Haude, Lukaskrankenhaus Neuss, Germany 2015; 10. Lipinski MJ, Acampado E, Cheng Q, et al.Comparison of Acute Thrombogenicity for Magnesium versus Stainless Steel Stents in a Porcine Arteriovenous Shunt Model. EuroIntervention. 2018 May 8. pii: EIJ-D-17-00958. doi: 10.4244/EIJ-D-17-00958; 11. Nakazawa G, Vorpahl M, Finn M, et al. One Step Forward and Two Steps Back With Drug-Eluting-Stents. JACC: Cardiovascular Imaging . 2009; 2(5): 625-628. doi: 10.1016/j.jcmg.2009.01.011; 12. Joner M. Systemic vs Site Targeted Treatment of Neoatherosclerosis. Presented at: ESC; Aug 28, 2017; Barcelona, Spain; 13. Andreou I, Stone P. In-Stent Atherosclerosis at a Crossroads. Neoatherosclerosis … or Paleoatherosclerosis? Circulation. 2016;134:1413–1415. doi: 10.1161/CIRCULATIONAHA.116.025129; 14. Bennett J. Safety and Efficacy of the Resorbable Magnesium Scaffold, Magmaris in a Real-World Setting – 24-month Follow-up of the Full Cohort (2066 subjects) of the BIOSOLVE-IV Registry. Presented at: TCT, September 2022, Boston, USA. ClinicalTrials.gov: NCT02817802
; 15. Torzewski J. Safety and performance of Magmaris at 36-months: BIOSOLVE-IV first cohort. Presented at: EuroPCR; 2022; ClinicalTrials.gov: NCT02817802; 16. Haude M, Toelg R , Lemos P.A et al. Sustained safety and performance of a second-generation sirolimus-eluting absorbable metal scaffold: Long-term data of the BIOSOLVE-II first-in-man trial at 5 years. Cardiovascular Revascularization Medicine. 2021. doi: 10.1016/j.carrev.2021.07.017; 16. Haude M, Toelg R , Lemos P.A et al. Sustained safety and performance of a second-generation sirolimus-eluting absorbable metal scaffold: Long-term data of the BIOSOLVE-II first-in-man trial at 5 years. Cardiovascular Revascularization Medicine. 2021. doi: 10.1016/j.carrev.2021.07.017; 17. Joner M. Magmaris: Reducing the risk of neoatherosclerosis. Presented at: TCT; Sep 22, 2018; San Diego, USA.
BIOSOLVE- II and -IV based on Kaplan-Meier failure estimate analysis including censored observations. The pooled analysis of BIOSOLVE-II and -III based on frequency analysis.
Magmaris is a trademark or registered trademark of the BIOTRONIK Group of Companies. Xience is a trademark or registered trademark of the Abbott Group of Companies. Synergy is a trademark or a registered trademark of the Boston Scientific Group of Companies.
Magmaris is currently not available in the US.
© 2022 BIOTRONIK AG – All rights reserved.
Specifications are subject to modification, revision and improvement.