Clinical Studies
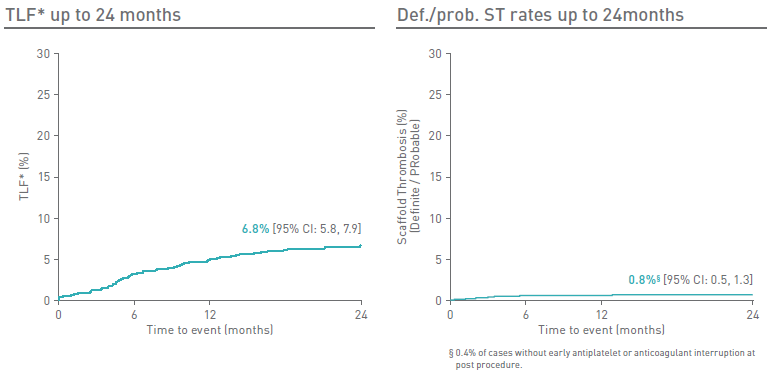
Magmaris® RMS’ clinical body of evidence keeps growing with new data from BIOSOLVE-IV of the full cohort with 2,066 patients at 24-month follow-up.

Magmaris® RMS’ clinical body of evidence keeps growing with new data from BIOSOLVE-IV of the full cohort with 2,066 patients at 24-month follow-up.
BIOSOLVE-IV Full cohort
Prof. Johan Bennett, UZ Leuven, Leuven, Belgium

Prof. Johan Bennett, UZ Leuven, Leuven, Belgium
1. Bennett J. Safety and Efficacy of the Resorbable Magnesium Scaffold, Magmaris in a Real-World Setting – 24-month Follow-up of the Full Cohort (2066 subjects) of the BIOSOLVE-IV Registry. Presented at: TCT, September 2022, Boston, USA. ClinicalTrials.gov: NCT02817802.
Magmaris is a trademark or registered trademark of the BIOTRONIK Group of Companies.
Magmaris is currently not available in the US.
© 2022 BIOTRONIK AG – All rights reserved.
Specifications are subject to modification, revision and improvement.